Safety Screening and Implant Review Process
Our Commitment to MRI Safety
CFMI dedicates itself to providing a safe research environment for all users and research participants. Our safety procedures are meticulously designed to protect participants, users, and staff at every stage of the imaging process, adhering to the safety guidelines of the American College of Radiology (ACR). Our team trains in the latest MRI safety practices, and we continuously update our protocols to meet or exceed clinical standards. We strive to deliver high quality imaging data for all of our users while first and foremost ensuring the care and safety of all of our participants.
Why MRI Safety Matters
Magnetic Resonance Imaging (MRI) involves powerful magnetic fields and requires strict adherence to safety protocols to prevent harm. Because of these unique hazards, it is essential to maintain and follow well-defined MRI safety standards and train everyone who works in CFMI in our safety standard operating procedure.
CFMI is a research-dedicated facility, not a clinical imaging center. This means that research participants do not receive direct medical benefit* from undergoing MRI. For that reason, we only conduct studies that pose no more than minimal risk for participants. Following a strict set of safety procedures is imperative to maintaining this classification as a minimal risk activity.
Core Principles of MRI Safety
- Understanding and mitigating risks associated with the three magnetic fields (static, gradient and RF).
- Performing careful, consistent participant screening.
- Throughly reviewing implants to ensure their safety.
- Restricting access to the MRI scanner, and ensuring direct supervision in controlled zones.
- Moving with intention in each of the access-restricted zones to prevent accidents.
- Responding effectively to emergency situations.
Failure to follow MRI safety protocols can result in serious injury or death. As such, violation of safety procedures may lead to the revocation of scanner access privileges.
MRI Safety Process at CFMI
This diagram outlines the step-by-step MRI safety screening process for research participants at CFMI:
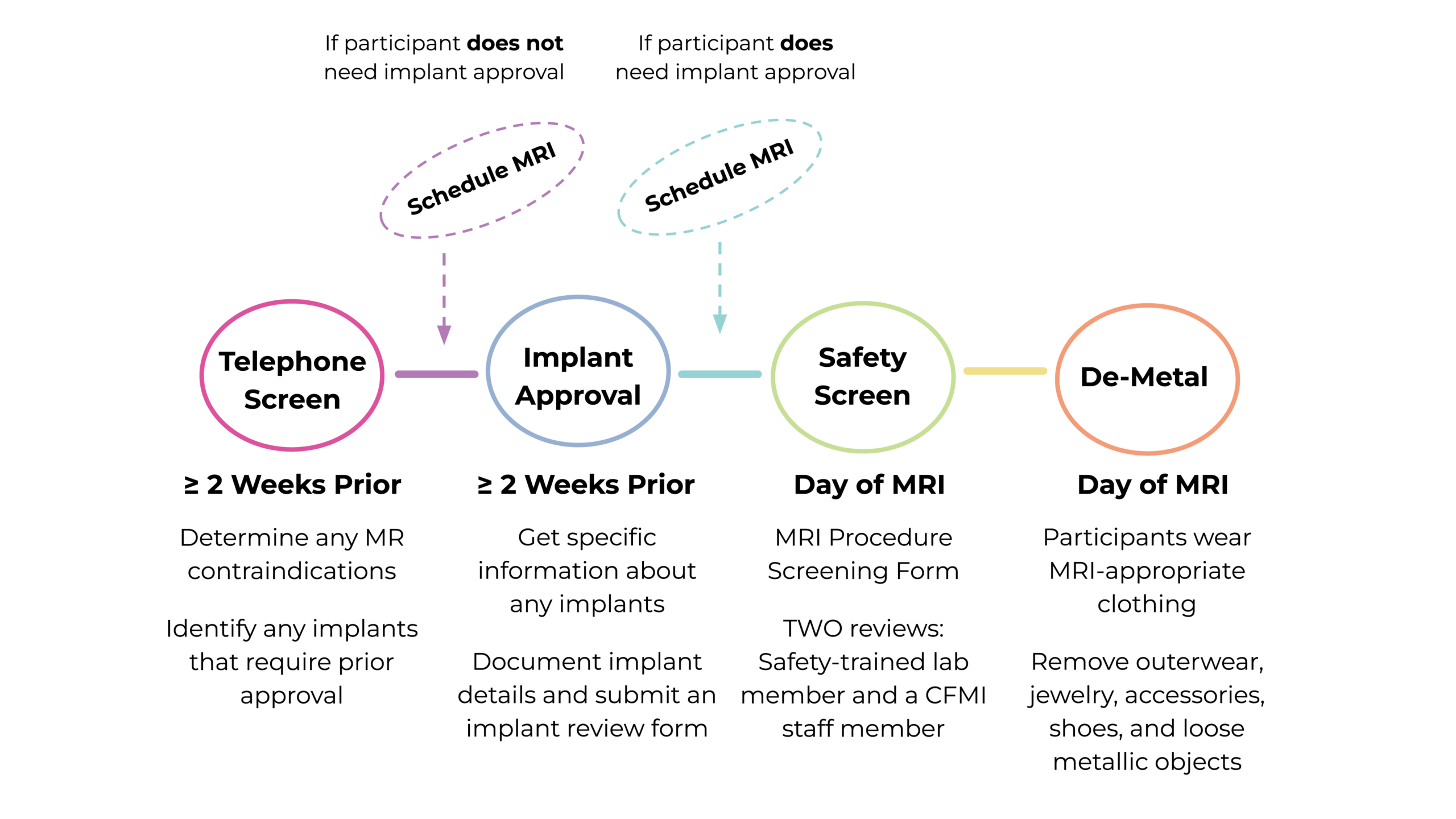
1. Telephone Screen (Pre-Screen)
Conduct a telephone screen (or pre-screening interview) with the participant at least 2 weeks before the potential scan date. During this screen, researchers should determine any MR contraindications and identify any known or potential implants that require approval prior to scanning.
- Examples of MR contraindications: Pacemakers, cochlear implants, inability to lie comfortably in the MRI scanner for any reason.
- Examples of surgeries/implants that require prior approval: Hip replacement, thyroid removal (thyroidectomy), stents, craniotomy, craniectomy, or cranioplasty.
Telephone Screening Resources:
- Telephone Screen Worksheet – a printable worksheet to aid researchers in telephone screening participants.
- Safety Terms Glossary – a list of common surgeries, procedures and implants, and their definitions.
2. Implant Review and Approval
During pre-screening, note any known implants or surgeries that commonly utilize implants. An implant review involves gathering detailed information about any implanted medical device a participant may have (e.g., stents, surgical clips, etc.). Not all implants are MRI-safe, and some require very specific scanning conditions.
Our Implant Approval Guide for Users is a list of common surgeries and implants meant to aid CFMI researchers in making decisions regarding participant safety. Please refer to this document when deciding the necessity of an implant review.
Note: Our safety documentation is constantly evolving. Please check this guide often for updates.
If an implant review is necessary, please complete the following steps:
3. Safety Screen
Participants must complete the MRI Procedure Screening Form within 24 hours of their appointment. They may complete a printable version or a digital version of the form. Blank, printed forms are also available in the CFMI waiting room.
All participants must undergo a two-step safety screening prior to their MRI scan:
- A first screen is conducted by a safety-trained researcher from the sponsoring research group.
- A second screen is then performed by a CFMI staff member.
When you sign off on a participant’s screening form, you are confirming that you have carefully reviewed each item on the form with the participant and have no concerns about their MRI safety. This signature indicates that, to the best of your knowledge, the participant meets all safety criteria for scanning.
4. De-Metal
Before entering the MRI room, you must guide your participant through the de-metal process to ensure their safety. This involves having them remove all metallic items, including jewelry (earrings, necklaces, bracelets, rings), watches, hairpins, bobby pins, hearing aids, orthotics, belts, wallets, phones, keys, and any other loose objects or accessories that may contain metal.
Participants should also empty their pockets and remove their shoes.
It’s essential to be thorough during de-metaling since even small metallic objects can pose serious safety risks or interfere with image quality. If you’re unsure about an item, consult CFMI staff before proceeding.
Final Reminders
MRI safety is a shared responsibility. Thoroughly following each step of the safety process ensures a safe and efficient scan experience for everyone at CFMI.
If you have any questions about the MRI screening process, implant reviews, or safety procedures, please contact us at cfmi-scanning-team@georgetown.edu. When in doubt, always ask — your caution helps protect everyone involved.
For other helpful information for ensuring a smooth scan day, visit our Scan Day Tips & Tricks page.